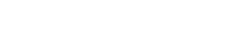Search Collagen Crew Pilot
Can We Measure Corneal Biomechanical ChangesAfter Collagen Cross-Linking in Eyes WithKeratoconus?—A Pilot Study
Yakov Goldich, MD, Yaniv Barkana, MD, Yair Morad, MD, Morris Hartstein, MD, Isaac Avni, MD, and David Zadok.
Purpose: To assess changes in biomechanical properties of human cornea after treatment of keratoconus with UV-A–riboflavin corneal collagen cross-linking (CXL).
Design: Single-center, prospective, interventional study.
Methods: Ten eyes of 10 patients aged 26.5 6 5.7 (mean 6 SD) years with progressive keratoconus were treated with UV-A– riboflavin CXL and assessed with the Ocular Response Analyzer (ORA) that measured corneal hysteresis (CH), corneal resistance factor (CRF), Goldmann-correlated intraocular pressure (IOPg), and corneal compensated intraocular pressure (IOPcc). Intraocular pressure was also measured by Goldmann applanation tonometry (GAT-IOP). Patients were assessed with ORA preoperatively, at week 1, months 1, 3, and 6 after treatment. Postoperative measurements at each visit were compared with preoperative values.
Results: CH and CRF were transiently elevated after cross-linking treatment, with the difference not statistically significant (P . 0.3). IOPcc and IOPg were statistically significantly higher at 1 week and 1 month but not subsequently (P , 0.04). GAT-IOP was statistically significantly higher at 1 week and at 1 and 3 months (P , 0.01).
Conclusions: There were no significant differences in corneal biomechanical properties, as measured with the ORA parameters CH and CRF, after CXL in keratoconus. IOPcc, IOPg, and GAT-IOP values were transiently elevated after CXL treatment in our study. Whether this reflects a measurement artifact resulting from corneal changes or true elevation of intraocular pressure is unclear.
Key Words: cross-linking, keratoconus, collagen, riboflavin–UV-A, Ocular Response Analyzer
(Cornea 2009;28:498–502)
Received for publication August 4, 2008; revision received October 3, 2008; accepted October 6, 2008.
From the Department of Ophthalmology, Assaf Harofeh Medical Center, Tel-Aviv University, Tel-Aviv, Israel.
No author has a financial or proprietary interest in any material or method mentioned.
Reprints: Yakov Goldich, MD, Department of Ophthalmology, Assaf Harofeh Medical Center, Beer Yaakov, Zerifin 70300, Israel (e-mail: doctor. goldich@gmail.com).Copyright 2009 by Lippincott Williams & Wilkins
498 | www.corneajrnl.com
Keratoconus is a progressive ectasia of the cornea resulting from noninflammatory thinning of the corneal stroma.1 Visual impairment from myopia and irregular astigmatism is commonly noted in adolescence and progresses thereafter.2 The initial management of keratoconus is based on refractive correction with spectacles and contact lenses. Further ectatic progression ultimately necessitates corneal transplantation in10%–20% of patients.3
Corneal collagen cross-linking (CXL) using UV-A and riboflavin was proposed recently as treatment to halt the progression of keratoconus.4 Others have proposed that similar cross-linking of collagen fibers occurs naturally in diabetic corneas and thus provides protection against the progression of keratoconus in patients with diabetes.5,6 Previous in vitro studies have shown increased corneal rigidity7–9 and increased corneal resistance to enzymatic degradation10 after CXL. In vivo evaluation of changes in biomechanical properties after human CXL treatment has not been described. The Ocular Response Analyzer (ORA; Reichert, Inc, Buffalo, NY) can be used to assess in vivo corneal biomechanical properties, presented by 2 parameters, corneal hysteresis (CH) and corneal resistance factor (CRF). It also provides noncontact measurement of intraocular pressure (IOP) through the param-eters Goldmann-correlated IOP (IOPg) and corneal compen-sated IOP (IOPcc). Detailed description of this instrument has been previously published.11 Briefly, the instrument measures corneal response to indentation by a rapid air pulse using an electro-optical system. The air puff causes the cornea to move inward, passing a defined point of applanation and into a slight concavity. After reaching the pressure peak, the pressure of the air pulse decreases and the cornea returns to its normal configuration, passing again the defined point of applanation. The electro-optical system monitors this entire process and calculates the above parameters. CH represents the absolute difference between the 2 pressure values causing force-in (P1) and force-out (P2) applanations and provides a measure of viscous damping of the cornea. The CRF is derived from the formula (P1 2 kP2), where k is a constant. The constant k was determined from an empirical analysis of the relationship between both P1 and P2 and central corneal thickness (CCT) to develop a parameter more strongly associated with CCT than CH.12 IOPg is the average of the 2 IOP measurements at the applanation points. IOPcc is a pressure measurement that uses the information provided by CH to provide an IOP that is less affected by CCT or corneal curvature.11
Cornea Volume 28, Number 5, June 2009
Copyright © 2009 Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
Cornea Volume 28, Number 5, June 2009 Measuring Corneal Biochemical Changes After CXL
The aim of our study was to prospectively assess in vivo the changes in biomechanical properties of human corneas with the ORA after treatment of keratoconus with UV-Ariboflavin CXL.
METHODS
Patients with keratoconus were prospectively recruited from the Cornea Outpatient Clinic of the Assaf Harofeh Medical Center. Inclusion criteria were progressive keratoco-nus documented clinically within the past 12 months by astigmatic refraction and/or topography, age over 18 years, no previous ocular surgery, no corneal opacities, minimal corneal thickness of 400 mm, and no wearing of contact lenses for 1 month before initial evaluation and treatment. Patients were treated with UV-A–riboflavin CXL under aseptic conditions using topical preoperative anesthesia with oxybuprocaine hydrochloride 0.4% drops (Localin; Fisher Pharmaceutical Labs). Treatment included 7-mm-diameter corneal deep-ithelization, instillation of 0.1% riboflavin in 20% dextran solution (Peschke Meditrade GmbH, Huenenberg, Switzer-land) every 5 minutes for 40 minutes, and corneal irradiation with UV-A 3 mW/cm2 (UV-X; Peschke Meditrade GmbH) for 30 minutes, 5 cm from the cornea. After the procedure, patients were treated with topical antibiotic (Oflox, ofloxacin 0.3%; Allergan) 4 times a day for 7 days, topical corticosteroid (Sterodex; dexamethasone 0.1%, Fisher Pharmaceutical Labs) 4 times a day for 1 month, and the eye was dressed with a soft therapeutic contact lens (Ocular Sciences, Ltd, Southampton, United Kingdom) for 3 days.
Patients were assessed before and at week 1, months 1, 3, and 6 after treatment. Each examination included measurement of best-corrected visual acuity, corneal topog-raphy, IOP by Goldmann applanation tonometry (GAT-IOP), slit-lamp and fundus examinations, and corneal biomechanical assessment using the ORA. We compared postoperative measurements at each visit with preoperative measurements.
For measurement with the ORA, each patient was seated and asked to fixate at a target light, and the measurement was taken by pressing a button on a personal computer linked to the ORA. A noncontact probe scans the central corneal area and releases an air puff. Measured IOP, CH, and CRF are displayed on the monitor. For each patient, we obtained 3 readings of good quality, defined as having a waveform with 2 distinct peaks, and recorded the average for each parameter.
This study was approved by the Institutional Ethics Committee of Assaf Harofeh Medical Center, and a written informed consent was obtained from each subject after the nature and intent of the study had been fully explained. The study protocol was consistent with the tenets of the Declaration of Helsinki.
Statistical Analysis
The data are presented as frequency or mean 6 standard deviation. Paired 2-tailed Student t test was used to assess differences between the compared groups in CH, CRF, CCT, IOPcc, IOPg, and GAT-IOP. The distributions of values within each data set were evaluated graphically. A P value of 0.05 was
q 2009 Lippincott Williams & Wilkins
selected for the threshold of statistical significance. Analyses were performed using Excel (Microsoft, Corp, Redmond, WA).
RESULTS
Ten eyes of 10 patients were treated with UV-A– riboflavin CXL and assessed prospectively with the ORA. There were 3 females and 7 males with mean age 26.5 6 5.7 years (range 18–37 years). Mean maximum keratometry
(Kmax) reading was 53.07 6 6.3 diopters, and mean CCT was 470 6 36.6 mm.
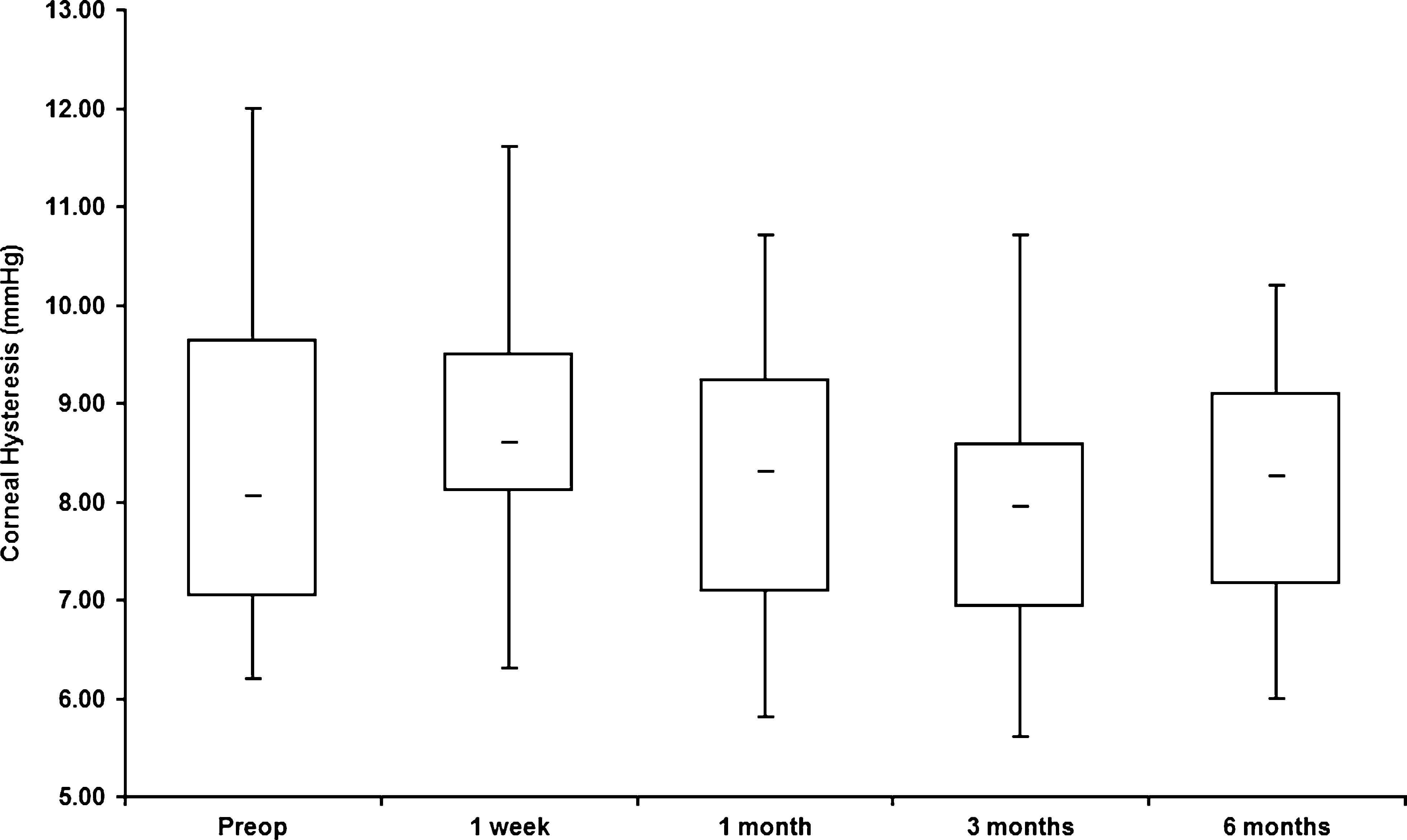
Table 1 presents the mean CH and CRF values before treatment and week 1, months 1, 3, and 6 after treatment. Mean CH and CRF values were transiently increased (at 1 week and 1 month) after CXL, with the differences from baseline not statistically significant. Figures 1 and 2 present the maximal, minimal, and median values with interquartile ranges of CH and CRF before and after CXL treatment.
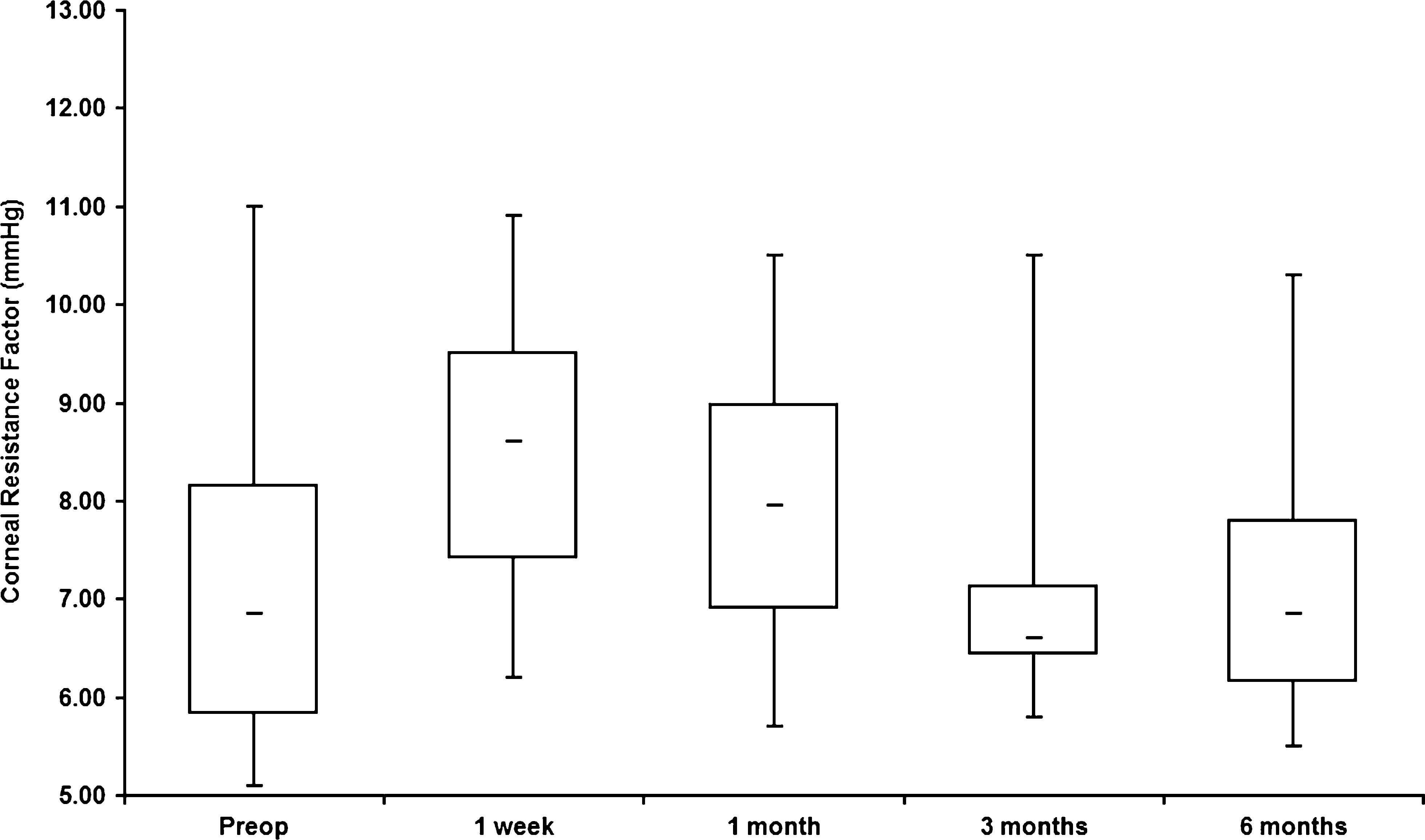
Mean IOPcc and IOPg were statistically significantly higher at 1 week and 1 month after CXL compared with preoperative values (Table 2, Fig. 3). Statistically significant differences between preoperative and postoperative values were found for GAT-IOP at 1 week, 1 month, and 3 months (Table 2).
DISCUSSION
Because cross-linking of collagen supposedly halts progression of keratoconus by increasing the stiffness of the cornea,4 we expected to observe changes in CH and CRF after treatment. Theoretically, therapeutically inducing cross-linking of collagen might act similarly to processes that retard keratoconus progression during aging and prolonged uncon-trolled hyperglycemia.5,6,13
In this pilot short-term study, we did not observe significant changes in biomechanical properties of the cornea after CXL for keratoconus as measured in vivo by ORA. It is noteworthy that the baseline values of CH and CRF in our study were similar to those reported in previous studies of keratoconic eyes.14,15
The effect of this treatment has been previously assessed only using clinical parameters such as corneal topography and subjective refraction,4,16 whereas we sought to demonstrate physical corneal changes. Previous in vitro studies described physical changes in the cornea after cross-linking. Wollensak et al8 used stress–strain measurements to evaluate the effect of riboflavin–UV-A CXL on corneal rigidity in human and
TABLE 1. Biomechanical Properties Before and AfterCXL Treatment Time CH CRF Before 8.44 6 1.82 7.15 6 1.771 wk 8.62 6 1.56 (P = 0.1) 8.48 6 1.55 (P = 0.82)1 mo 8.22 6 1.50 (P = 0.77) 7.91 6 1.54 (P = 0.32)3 mo 7.88 6 1.57 (P = 0.46) 7.1 6 1.51 (P = 0.94)6 mo 8.14 6 1.32 (P = 0.32) 7.16 6 1.45 (P = 0.87).
www.corneajrnl.com | 499 Copyright © 2009 Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
Goldich et al Cornea Volume 28, Number 5, June 2009
FIGURE 1. Box and whisker plots (smallest, median, and largest values with interquartile range) showing CH before treatment (pre-operative) and on 1 week, 1 month, 3 months, and 6 months thereafter.
porcine corneas. Using microcomputer-controlled biomaterial tester, they showed significant increase in rigidity in both human and porcine corneas. Dupps et al9 used an ultrasonic device to evaluate the effect of human and porcine corneal cross-linking with glutaraldehyde. Through measuring sonic wave propagation time between 2 transducers positioned on the corneal surface, they found increased corneal stiffening after glutaraldehyde CXL. Noguera et al described an in vitro model of porcine cornea evaluation with ORA after CXL.17 In their study, significant elevation in both CH and CRF was seen in the subgroup treated with riboflavin–UV-A.
Our results, namely, the lack of biomechanical corneal changes after CXL, are in disagreement with the in vitro changes mentioned above. This may be explained, in part, by the different methodologies used to assess these changes and by some inherent differences between in vivo and in vitro.
FIGURE 2. Box and whisker plots (smallest, median, and largest val-ues with interquartile range) show-ing CRF before treatment (preoperative) and on 1 week, 1 month, 3 months, and 6 months thereafter. models. Additionally, it is plausible that biomechanical changes did occur but were too subtle to be measured by ORA.
One possible weakness of our study is the relatively short follow-up time. However, it has been shown in vivo15,16 that after riboflavin–UV-A CXL treatment, stromal keratocyte repopulation was complete by 6 months and was accompanied by disappearance of stromal edema. Therefore, we think that it is unlikely that a greater amount of change would be observed with longer follow-up. Another weakness is the small number of participants. Because our pilot study included a small number of eyes, we cannot rule out that statistically significant changes could be demonstrated with a larger sample size. Further study is needed in this regard.
Recently, more advanced analysis of the raw data provided by ORA has been suggested in addition to the CH and CRF parameters in biomechanical evaluation. Thisq 2009 Lippincott Williams & Wilkins.
Copyright © 2009 Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
Cornea Volume 28, Number 5, June 2009 Measuring Corneal Biochemical Changes After CXL TABLE 2. Intraocular Pressure Before and After CXL Treatment Time IOPcc IOPg GAT-IOP Before 13.6 6 2.06 10.2 6 1.63 10.1 6 1.66 1 wk 16.7 6 2.40 (P = 0.01) 14.1 6 2.21 (P , 0.001) 14.2 6 2.73 (P , 0.001)1 mo 16.5 6 3.57 (P = 0.04) 13.2 6 3.55 (P = 0.03) 13.5 6 3.12 (P = 0.01)3 mo 15.3 6 3.48 (P = 0.19) 11.6 6 3.17 (P = 0.23) 12.0 6 1.25 (P = 0.01)6 mo 14.7 6 2.87 (P = 0.21) 11.2 6 2.89 (P = 0.33) 12.2 6 1.14 (P = 0.16)
analysis is performed using graphically presented waves and includes comparison of signal peak amplitudes and shape,19 width of infrared peaks at their mid-height point, and slope of air pulse during the 2 applanation events.20 Once the accuracy and reliability of these analytic tools are established, they can potentially be used to reexamine the effect of CXL.
Various factors might influence the measurement of postoperative IOP including corneal biomechanical properties, corneal thickness, and corneal curvature.21–24 The ORA permits in vivo noncontact evaluation of pressure metrics through IOPcc and IOPg. Noncontact IOP evaluation by ORA was examined in previous studies.23,25,26 Martinez-de-la-Casa et al26 and Broman et al23 showed that IOP values in glauco-matous eyes as measured by the ORA were higher, on average, than values measured with Goldmann applanation tonometer. The relationship between ocular characteristics and interaction of different types of tonometers with the eye is complex, although IOPcc seems to provide estimation of IOP that is less influenced by corneal properties than Goldmann tonometer.11,25
In our study, IOP measurements were taken by ORA and Goldmann tonometer on every follow-up visit. GAT-IOP values up to 3 months postoperatively were statistically significantly higher compared with preoperative values. A statistically significant elevation of noncontact IOP measure-ments (IOPcc and IOPg) was detected 1 week and 1 month after treatment. Whether these changes reflect true elevation
FIGURE 3. Box and whisker plots (smallest, median and largest values with interquartile range) showing preoperative IOP (IOPcc preopera-tive and IOPg preoperative) and corresponding values of both IOPcc and IOPg on 1 week, 1 month, 3 months, and 6 months after cross-linking treatment.
q 2009 Lippincott Williams & Wilkins
of IOP is uncertain. Previously, Wollensak et al4 found no significant change in IOP after CXL measured with the Goldmann tonometer. In the above mentioned study by Dupps et al,9 it was reported that IOP measurements taken with TonoPen in vitro, after CXL with glutaraldehyde, were higher despite keeping constant IOP. They presumed that stiffening of the human cornea is responsible for such artifactual increase in measured IOP. The Siena Study Group,16,18 by using confocal microscopy, described transient stromal edema appearing shortly after riboflavin–UV-A CXL and disappearing by 3 months. They also reported increased CCT measured by ultrasonic pachymetry after CXL but without significant IOP elevation as measured by TonoPen. Thus, early postoperative IOP changes in our study may be a measurement artifact following changes in ocular characteristics, like increased corneal thickness, and not a real IOP elevation. Because the increased IOP was observed as early as 1 week after CXL, we think it unlikely that it is secondary to response to topical steroid treatment, but this cannot be ruled out.
In conclusion, in the present study, we did not observe significant change in corneal biomechanical properties, as measured with the ORA parameters CH and CRF, after CXL in keratoconus. Measured IOP values were transiently elevated after CXL treatment in our study. Whether this reflects a measurement artifact resulting from corneal changes or true elevation of IOP is unclear.
Copyright © 2009 Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
Goldich et al Cornea Volume 28, Number 5, June 2009
REFERENCES
1. Krachmer JH, Feder RS, Belin MW. Keratoconus and related non-inflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28: 293–322.
2. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319.
3. Tuft SJ, Moodaley LC, Gregory WM, et al. Prognostic factors for the progression of keratoconus. Ophthalmology. 1994;101:439–447.
4. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135:620–627.
5. Kuo IC, Broman A, Pirouzmanesh A, et al. Is there an association between diabetes and keratoconus? Ophthalmology. 2006;113:184–190.
6. Seiler T, Huhle S, Spoerl E, et al. Manifest diabetes and keratoconus: a retrospective case-control study. Graefes Arch Clin Exp Ophthalmol. 2000;238:822–825.
7. Kohlhaas M, Spoerl E, Schilde T, et al. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–283.
8. Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human andporcine corneas after riboflavin-ultraviolet-A-induced cross-linking.J Cataract Refract Surg. 2003;29:1780–1785.
9. Dupps WJ Jr, Netto MV, Herekar S, et al. Surface wave elastometry of the cornea in porcine and human donor eyes. J Refract Surg. 2007;23: 66–75.
10. Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40.
11. Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31: 156–162.
12. Luce D. Methodology for corneal compensated IOP and corneal resistance factor for the Reichert ocular response analyzer. IOVS. 2006;47:ARVO. E-Abstract 2266.
13. Daxer A, Misof K, Grabner B, et al. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci. 1998;39:644–648.
14. Shah S, Laiquzzaman M, Bhojwani R, et al. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci. 2007;48: 3026–3031.
15. Ortiz D, Pinero D, Shabayek MH, et al. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33:1371–1375.16. Caporossi A, Baiocchi S, Mazzotta C, et al. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–845.
17. Noguera G. Ocular response Analyser uses to measure corneal biomechanics. IOVS. 2007;48:ARVO. Poster 1860/B931.
18. Mazzotta C, Balestrazzi A, Traversi C, et al. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal colla-gen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26:390–397.
19. Kerautret J, Colin J, Touboul D, et al. Biomechanical characteristics of the ectatic cornea. J Cataract Refract Surg. 2008;34:510–513.
20. Glass D. Evaluation of the deformation response to an air puff in healthy and diseased in vivo human corneas. IOVS. 2008:49ARVO. Poster 646/ D927.
21. Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–155.
22. Bhan A, Browning AC, Shah S, et al. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol Vis Sci. 2002; 43:1389–1392.
23. Broman AT, Congdon NG, Bandeen-Roche K, et al. Influence of corneal structure, corneal responsiveness, and other ocular parameters on tonometric measurement of intraocular pressure. J Glaucoma. 2007;16:581–588.
24. Feltgen N, Leifert D, Funk J. Correlation between central corneal thickness, applanation tonometry, and direct intracameral IOP readings. Br J Ophthalmol. 2001;85:85–87.
25. Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J Glaucoma. 2006;15:364–370.
26. Martinez-de-la-Casa JM, Garcia-Feijoo J, Fernandez-Vidal A, et al. Ocular response analyzer versus Goldmann applanation tonometry for intraocular pressure measurements. Invest Ophthalmol Vis Sci. 2006;47: 4410–4414.
Copyright © 2009 Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.